June 2, 2016
Finally we have made it to week 10, the final week of the freshman design class.
Over the past week we have been preparing as a group for our final report and presentation.
For the report, we took what we had from the draft and added in our results from the third and most successful trial. We included that trial in the analysis, and showed how the rate of heat transfer and the temperature gap at equilibrium were much better than in the first two trials. We were also sure to mention how the success of the third trial fit into the larger scope of our goals for the lab.
We also took these adaptations to the blog, commenting on how we felt we stuck to the timeline and project goals throughout the course of the ten weeks. Those comments can be found on the goals and timeline pages respectively on our blog.
For the presentation, we used our final report as a guide to pick out the most important points from our experience building the heat pipe. We made sure to touch on our goals, data, equations, and what we learned as a result of planning and execution.
After presenting our project to the class, we felt that it was a tremendous success. Everyone was able to hit on their key talking points and kept the class engaged through demonstrations and animations. While the layout of our slides could have been improved, we were able to completely and concisely answer the questions from the class.
All in all, our group felt that our project was a great success. We had a wonderful experience planning and testing our project, and feel that it is something we can take into the future as we prepare for work on real world engineering problems.
-- Alec, Tran, Matt, and Shjon
Group 067-06: Heat Pipes
A week by week update on our freshman design project to build a copper/water heat pipe
Thursday, June 2, 2016
Thursday, May 26, 2016
Week 9
May 26, 2016
In recent weeks, we had made great progress towards completing all of the refinements to our design.
To start week 9, we sat down as a group and gathered all of our data and results. We recognized that trials 1 and 2, where the fluid level was constant but the wick size was changed, would provide comparable data to conclude which wick (larger or smaller) allowed for a more efficient and effective heat pipe.
Trial 3 was a success as well, as we used the more effective wick and tinkered with the fluid level within the pipe. By reaching an equilibrium during which the temperatures were effectively equal, we had refined our design to the point of achieving success. We used this trial to compare to the first two, to show how both wick size and fluid levels had an impact on the overall efficiency.
After we had gathered our data and compared results, we used various equations to describe the relationships present. These equations are presented with more detail under the equations tab at the top of this page. In the end, after accounting for the diameter of the screen wires and the width of the holes in the screen, we were able to calculate values of wick permeability for each screen.
By simplifying known equations, we derived a proportionality between wick permeability and fluid friction. We showed that fluid friction was directly related to wick permeability. Since the 16 hpi wick had a greater permeability, it therefore exhibited greater internal fluid friction. A greater amount of friction can slow down the movement of condensed water, and would thus create a less efficient heat pipe. This ultimately agreed with our results from trials 1 and 2, which showed that the 16 hpi was less effective.
The rest of week 9 was spent creating and practicing our final presentation. A final copy of this presentation will be available below after it is finalized. Through much practice, this presentation will allow us to tell the story of our project, what we learned, and how it could have been improved.
On the whole, we feel as if we were very successful in the goals we set out to achieve. Through a three week literature study, we were able to find the information needed to choose the best materials for the project. This literature study also allowed us to identify the gaps in prior research, such as testing with cheap metal mesh wicks, and focus our analysis accordingly. Over the course of many weeks of refining and changing our heat pipe, we ultimately landed on an effective design that could be used and applied in a real world cooling application. In the end, we were able to establish a relationship between wick size and effectiveness of heat transfer, and had extra time to further refine the fluid levels within the pipe. By completing all of the goals we set out to achieve, as well as creating a working prototype, we feel that the project was an overall success.
We look forward to outlining our trial 3 results in the final report and presenting our work to students and faculty in week 10.
-- Alec, Tran, Matt, and Shjon
In recent weeks, we had made great progress towards completing all of the refinements to our design.
To start week 9, we sat down as a group and gathered all of our data and results. We recognized that trials 1 and 2, where the fluid level was constant but the wick size was changed, would provide comparable data to conclude which wick (larger or smaller) allowed for a more efficient and effective heat pipe.
Trial 3 was a success as well, as we used the more effective wick and tinkered with the fluid level within the pipe. By reaching an equilibrium during which the temperatures were effectively equal, we had refined our design to the point of achieving success. We used this trial to compare to the first two, to show how both wick size and fluid levels had an impact on the overall efficiency.
After we had gathered our data and compared results, we used various equations to describe the relationships present. These equations are presented with more detail under the equations tab at the top of this page. In the end, after accounting for the diameter of the screen wires and the width of the holes in the screen, we were able to calculate values of wick permeability for each screen.
By simplifying known equations, we derived a proportionality between wick permeability and fluid friction. We showed that fluid friction was directly related to wick permeability. Since the 16 hpi wick had a greater permeability, it therefore exhibited greater internal fluid friction. A greater amount of friction can slow down the movement of condensed water, and would thus create a less efficient heat pipe. This ultimately agreed with our results from trials 1 and 2, which showed that the 16 hpi was less effective.
The rest of week 9 was spent creating and practicing our final presentation. A final copy of this presentation will be available below after it is finalized. Through much practice, this presentation will allow us to tell the story of our project, what we learned, and how it could have been improved.
On the whole, we feel as if we were very successful in the goals we set out to achieve. Through a three week literature study, we were able to find the information needed to choose the best materials for the project. This literature study also allowed us to identify the gaps in prior research, such as testing with cheap metal mesh wicks, and focus our analysis accordingly. Over the course of many weeks of refining and changing our heat pipe, we ultimately landed on an effective design that could be used and applied in a real world cooling application. In the end, we were able to establish a relationship between wick size and effectiveness of heat transfer, and had extra time to further refine the fluid levels within the pipe. By completing all of the goals we set out to achieve, as well as creating a working prototype, we feel that the project was an overall success.
We look forward to outlining our trial 3 results in the final report and presenting our work to students and faculty in week 10.
-- Alec, Tran, Matt, and Shjon
Thursday, May 19, 2016
Week 8
May 19, 2016
In week 8 we had some great success with our third (and likely final) round of testing. The changes that we made to the pipe over the course of the previous weeks allowed the pipe to operate wit high efficiency, with the temperatures at both ends of the pipe being roughly equal as the system reached a state of equilibrium.
We began week 8 by testing our existing design. This was the design from the previous week, where we filled the pipe 35% of the way with water and used the 22 hpi wick (the smallest of the two).
We learned from trials 1 and 2 that there was a relationship between wick size (hpi) and efficiency of heat transfer. The smaller the wick size, the greater the capillary action the wick could provide to the condensing water, which translates to a more efficient heat pipe.
Additionally, from initial testing we learned that filling 25% of the pipe with working fluid was not enough to transfer heat all of the way to the cool end of the pipe. In trials 1 and 2, the pipe worked with greater efficiency as we filled it 50% of the way with water. However, from additional research, we hypothesized that reducing the amount of working fluid to some "happy medium" between 25% and 50% of the pipe's total volume would allow for maximum efficiency of heat transfer. We chose 35% to start, and planned to adjust this value in either direction as needed after examining the data from trial 3.
Just as a reminder here is a list of the changes made from trial to trial
We initially thought that we would have to adjust the working fluid after Trial 3, however additional adjustments were not needed, as the data from trial 3 showed that the pipe operated near maximum efficiency. Below is the graph in the same format as before, with blue representing the temperature at the heated end of the pipe and orange showing the temperature at the cooler end of the pipe.
In week 8 we had some great success with our third (and likely final) round of testing. The changes that we made to the pipe over the course of the previous weeks allowed the pipe to operate wit high efficiency, with the temperatures at both ends of the pipe being roughly equal as the system reached a state of equilibrium.
We began week 8 by testing our existing design. This was the design from the previous week, where we filled the pipe 35% of the way with water and used the 22 hpi wick (the smallest of the two).
We learned from trials 1 and 2 that there was a relationship between wick size (hpi) and efficiency of heat transfer. The smaller the wick size, the greater the capillary action the wick could provide to the condensing water, which translates to a more efficient heat pipe.
Additionally, from initial testing we learned that filling 25% of the pipe with working fluid was not enough to transfer heat all of the way to the cool end of the pipe. In trials 1 and 2, the pipe worked with greater efficiency as we filled it 50% of the way with water. However, from additional research, we hypothesized that reducing the amount of working fluid to some "happy medium" between 25% and 50% of the pipe's total volume would allow for maximum efficiency of heat transfer. We chose 35% to start, and planned to adjust this value in either direction as needed after examining the data from trial 3.
Just as a reminder here is a list of the changes made from trial to trial
Initial Testing: 25% water, 22 hpi wick
Trial 1: 50% water, 22 hpi wick
Trial 2: 50% water, 16 hpi wick
Trial 3: 35% water, 22 hpi wick
Below is a picture of the experimental setup. Due to technology errors these were not included in a previous blog post.
The temperature sensor is pictured in yellow. Notice how one probe was collecting the temperature near the heated end and the other probe at the cooler end. The heat pipe was secured in a temperature resistant rubber clamp, and kept at a constant angle across all trials. The hot air blower is shown in red, with the air directed towards the clear heat shield and the fixed cap end of the pipe. Not pictured is the timer and notebook, in which the temperature at each probe was recorded in 30 second intervals.
We initially thought that we would have to adjust the working fluid after Trial 3, however additional adjustments were not needed, as the data from trial 3 showed that the pipe operated near maximum efficiency. Below is the graph in the same format as before, with blue representing the temperature at the heated end of the pipe and orange showing the temperature at the cooler end of the pipe.
Using the same methods of analysis detailed in the week 6 post, the rate of heat transfer was calculated to be 0.136 °F/s. This analysis was done on the curved portion of the graph from t = 390s to t = 780s, which represents the time during which the working fluid was evaporating and condensing to influence heat transfer.
Not only was this rate of heat transfer far greater than any rate calculated from previous trials, but an equilibrium was established very close to the temperature at the heated end of the pipe. The cooler end of the pipe got within 6 °F of the heated end, representative of the success with which the working fluid was able to evaporate and condense using the wick to transfer heat away from the source. While ideally these two temperatures would be equal at equilibrium, we were quite happy with how close we were able to get them in a laboratory setting.
Going forward, we will be continuing with the analysis of trial 3 as well as the comparison between all three of the trials conducted. We also plan to go back and look at how our thought process and hypotheses evolved as we encountered different problems and obstacles from week to week. This reflection should influence our final presentation in week 10, where we can detail not only the results of the project but what we learned that will help us in a future setting at professional engineers.
We look forward to start on the final analysis and presentation going into the last two weeks of the term.
-- Alec, Tran, Matt, and Shjon
Thursday, May 12, 2016
Week 7
May 12, 2016
In week 7 we continued to work towards improving and refining the current heat pipe design.
After completing the analysis of the data from both trials (as shown in the final report and the analysis section of the blog), we noticed that smaller wicks displayed greater capillary action, which allowed for greater rates of heat transfer. However, even with using our smallest available wick (22 hpi), the equilibrium temperature of the cool end of the pipe was roughly 40°F less than the temperature at the warm end of the pipe.
From this we thought of two ways that the pipe could be improved so that the temperatures at both end of the pipe could become roughly equal, at which point the heat pipe would be operating at maximum efficiency.
Possibility 1: change the amount of working fluid present in the pipe
From careful literature study and analysis of the merit numbers (see analysis section) for various types of working fluids, we already knew that water would be the optimal fluid to use in the final design. What we didn't know, however, was how much of it should be used.
Theoretically, we wanted to use just enough so that the heat pipe would strike a balance between condensed fluid and vaporized fluid. We wanted the rate at which water was vaporizing inside the pipe to equal the rate at which it was condensing.
Our initial design was filled 25% of the way with water. This was definitely too little, as our heat pipe did not work as intended. During that trial we noticed that the middle of the pipe was reaching equilibrium, but not all the way to the cooler end. Because of this, we knew that the water was condensing before it reached the other end of the pipe, so we needed to add more.
Our past two trials have been conducted using a heat pipe filled slightly less than 50% of the way with water. While there was now sufficient water for the heat pipe to properly work, the equilibrium temperatures of both ends of the pipe were vastly different.
This led us to begin the week 7 lab by reducing the amount of working fluid in the pipe to 35% of the total pipe volume. We believed that we hit upon the two extremes with our first and second sets of trials, so we chose to fill it to a level in about the middle of that range.
We went through the same process of opening the pipe, draining then refilling the water, heating until vapor was observed, and securing the cap in place. Due to the large number of groups testing their pipes, we did not have time to test out our pipe with this configuration. Next week we plan to test it and analyze the results, to determine if our change in the amount of working fluid positively or negatively impacted the performance of the pipe. From those results we will be able to deliberate on further changes to be made to the design.
Even with the change to the working fluid, we still wondered if that would be enough to allow the heat pipe to operate as efficiently as possible, so we also thought of a second possible change.
Possibility 2: change the exposed surface area of the cooler end of the pipe
When the internal water vapor transports energy in the form of heat from the heated end to the cooler end, the cooler end starts to heat up. Eventually, the cooler end heats to the point where it no longer is cold enough to condense the water vapor.
The solution to this problem would be to increase the surface area of the cooler end of the pipe. An increased surface area would provide more area for the heat to spread out and dissipate into the surrounding environment. Since the cooler end of the pipe will thus stay cooler for longer, it can take on a larger amount of heat, allowing equal temperatures to be established between the heated and cooler ends.
This solution could be implemented by securing metal fins to the cooler end. These fins can be either crude sheets of metal or professionally manufactured. Either way, by securing fins onto the cooler end of the pipe, the surface area will be increased and hopefully a closer equilibrium between the two ends can be established.
For the time being we are focusing on possibility 1, changing the amounts of working fluid. After testing and analyzing our results from next week, we will be more certain as to whether we should continue to make changes to the amount of working fluid or if we should proceed with securing and testing the metal fins.
We look forward to continuing to optimize our heat pipe design with high hopes that we can get it to operate near maximum efficiency.
-- Alec, Tran, Matt, and Shjon
In week 7 we continued to work towards improving and refining the current heat pipe design.
After completing the analysis of the data from both trials (as shown in the final report and the analysis section of the blog), we noticed that smaller wicks displayed greater capillary action, which allowed for greater rates of heat transfer. However, even with using our smallest available wick (22 hpi), the equilibrium temperature of the cool end of the pipe was roughly 40°F less than the temperature at the warm end of the pipe.
From this we thought of two ways that the pipe could be improved so that the temperatures at both end of the pipe could become roughly equal, at which point the heat pipe would be operating at maximum efficiency.
Possibility 1: change the amount of working fluid present in the pipe
From careful literature study and analysis of the merit numbers (see analysis section) for various types of working fluids, we already knew that water would be the optimal fluid to use in the final design. What we didn't know, however, was how much of it should be used.
Theoretically, we wanted to use just enough so that the heat pipe would strike a balance between condensed fluid and vaporized fluid. We wanted the rate at which water was vaporizing inside the pipe to equal the rate at which it was condensing.
Our initial design was filled 25% of the way with water. This was definitely too little, as our heat pipe did not work as intended. During that trial we noticed that the middle of the pipe was reaching equilibrium, but not all the way to the cooler end. Because of this, we knew that the water was condensing before it reached the other end of the pipe, so we needed to add more.
Our past two trials have been conducted using a heat pipe filled slightly less than 50% of the way with water. While there was now sufficient water for the heat pipe to properly work, the equilibrium temperatures of both ends of the pipe were vastly different.
This led us to begin the week 7 lab by reducing the amount of working fluid in the pipe to 35% of the total pipe volume. We believed that we hit upon the two extremes with our first and second sets of trials, so we chose to fill it to a level in about the middle of that range.
We went through the same process of opening the pipe, draining then refilling the water, heating until vapor was observed, and securing the cap in place. Due to the large number of groups testing their pipes, we did not have time to test out our pipe with this configuration. Next week we plan to test it and analyze the results, to determine if our change in the amount of working fluid positively or negatively impacted the performance of the pipe. From those results we will be able to deliberate on further changes to be made to the design.
Even with the change to the working fluid, we still wondered if that would be enough to allow the heat pipe to operate as efficiently as possible, so we also thought of a second possible change.
Possibility 2: change the exposed surface area of the cooler end of the pipe
When the internal water vapor transports energy in the form of heat from the heated end to the cooler end, the cooler end starts to heat up. Eventually, the cooler end heats to the point where it no longer is cold enough to condense the water vapor.
The solution to this problem would be to increase the surface area of the cooler end of the pipe. An increased surface area would provide more area for the heat to spread out and dissipate into the surrounding environment. Since the cooler end of the pipe will thus stay cooler for longer, it can take on a larger amount of heat, allowing equal temperatures to be established between the heated and cooler ends.
This solution could be implemented by securing metal fins to the cooler end. These fins can be either crude sheets of metal or professionally manufactured. Either way, by securing fins onto the cooler end of the pipe, the surface area will be increased and hopefully a closer equilibrium between the two ends can be established.
For the time being we are focusing on possibility 1, changing the amounts of working fluid. After testing and analyzing our results from next week, we will be more certain as to whether we should continue to make changes to the amount of working fluid or if we should proceed with securing and testing the metal fins.
We look forward to continuing to optimize our heat pipe design with high hopes that we can get it to operate near maximum efficiency.
-- Alec, Tran, Matt, and Shjon
Thursday, May 5, 2016
Week 6
May 5, 2016
In week 6 we made substantial progress towards collecting the data we need to write and develop our analysis.
We began by testing our modified heat pipe. If you can recall from the previous week, we increased the amount of working fluid in the pipe, from 25% to 50%, in hopes that it would be sufficient to establish an equilibrium between evaporating and condensing fluid.
If too little fluid was in the pipe, like it was during our first test in week 4, the water would condense before it reached the top end of the pipe, which wouldn't allow for heat transfer over the entire length of the pipe. Too little working fluid would also run the risk of all of the fluid evaporating, which would render the heat pipe entirely ineffective.
If too much fluid was in the pipe, the vapor pressure in the empty pipe portion would be higher, not allowing for much fluid to evaporate. This would also render the heat pipe ineffective, since the fluid cannot evaporate and transfer heat away from the source.
Remember, the goal of our project was to create a working heat pipe then conduct a wick analysis comparing the rate of heat transfer of three differently sized wicks. We were not as concerned about our heat pipe operating at maximum efficiency, we just needed to ensure that it was working before proceeding with other tests on different wicks. Since we only had a limited number of tests we could conduct due to time constraints with the testing apparatus, it was more important to keep fluid levels and pipe orientation constant across all trials (in order to create a legitimate analysis) rather than proceeding with future modifications to improve general efficiency.
Trial 1 was conducted using a wick with 22 holes per inch (22 hpi). The blue curve shows the temperature of the heated end of the pipe as a function of time. The orange curve shows the recorded date for the cooler (higher) end of the pipe).
After the first trial was complete, we replaced the old wick with one of a different size. Fluid was completely drained from the pipe and carefully measured and refilled.
When the old wick was removed, many of its capillaries were saturated with water. We could not possibly know how much water was being lost, so we decided to completely drain the pipe and refill it with new working fluid, to ensure near constant conditions from trial to trial.
Careful note was also made as to the angle of inclination of the pipe during testing, to ensure that it could be returned to this angle for future trials.
We finished off the new pipe by taping the threads with PTFE tape and again heating it from the bottom until vapor exited from the open end. At this point the cap was quickly secured and the new pipe was ready for testing.
Trial 2 was conducted in a similar fashion using a slightly larger wick with 16 holes per inch (16 hpi).
The rate of heat transfer for each wick can be found by analyzing the curved portion of each graph. The linear portion before the curve reflects a period during which most of the water is condensed, and little is vaporized and contributing to the transfer of heat. The linear portion after the curve is a state of equilibrium between the transport of heat to the cooler (higher) end of the pipe and heat loss to the environment. If the experiment were left to continue past 720 seconds, this line would remain nearly horizontal, as the system is in balance between the upwards transfer of heat and the release of heat to the environment.
We observed the curved portion of the graph from trial 1 and noticed that it spanned the range t = 300s to t = 570s. We used the below equation to calculate the average rate of heat transfer.
We found that the rate of heat transfer for trial 1 with the 22 hpi wick was 0.085°F/s. The same analysis was done for trial 2 and the 16 hpi wick and we found that the rate of heat transfer was much lower, at 0.052°F/s.
This finding confirmed our hypothesis that a smaller wick size would result in a greater rate of heat transfer. The smaller wick provided better capillary action to efficiently return condensed water to the heated end of the pipe for re-evaporation.
We also noticed that the equilibrium temperature for trial 1 was 102°F, 10 degrees higher than the equilibrium temperature for trial 2, which was 92°F. This bolsters our belief that the smaller wick resulted in a more efficient heat pipe, since a greater amount of heat was able to be transferred to the cooler end of the pipe.
We finished by reflecting on some possible sources of error for our experiment. We immediately went to the temperature probes, and noticed that they did not maintain the same amount of contact from one trial to the next. It was difficult to keep the same surface area of the probe in contact with the curved edge of the pipe, which likely skewed some of the temperature readings. We also thought about the wick, and how it could have become bent or deformed when it was inserted into the pipe. Any tight bends or crimps could have negatively affected the ability of the wick to transport condensed water.
All in all, week 6 was a big success, with a few mistakes that we can learn from and improve upon. We were able to collect data for two different sized wicks and graph temperature vs time to start constructing a relationship between rate of heat transfer and wick size (holes per inch). However, due to a few external variables, conditions were not constant from trial to trial and likely caused our analysis to be slightly skewed.
Now that we are reaching the conclusion of the project, we have reassessed the project budget and materials list and noticed that the flux, solder, pipe cutter, and one roll of screen, as well as some extra caps and fittings were not used for the heat pipe. These additional materials have been returned, and our savings reflected in our finalized budget below.
By borrowing many materials that we needed to complete the pipe, we were able to save roughly $80.00 off of our original estimate of $119.54.
Heading into week 7, we look forward to potentially conducting a third test with a larger sized wick, to obtain a better idea of how wick size relates to heat transfer. We hope to learn from our mistakes and keep the external variables nearly constant to the other two trials for this upcoming test. We are eager to get started on drafting our final report and analysis for the upcoming week.
-- Alec, Tran, Matt, and Shjon
In week 6 we made substantial progress towards collecting the data we need to write and develop our analysis.
We began by testing our modified heat pipe. If you can recall from the previous week, we increased the amount of working fluid in the pipe, from 25% to 50%, in hopes that it would be sufficient to establish an equilibrium between evaporating and condensing fluid.
If too little fluid was in the pipe, like it was during our first test in week 4, the water would condense before it reached the top end of the pipe, which wouldn't allow for heat transfer over the entire length of the pipe. Too little working fluid would also run the risk of all of the fluid evaporating, which would render the heat pipe entirely ineffective.
If too much fluid was in the pipe, the vapor pressure in the empty pipe portion would be higher, not allowing for much fluid to evaporate. This would also render the heat pipe ineffective, since the fluid cannot evaporate and transfer heat away from the source.
Remember, the goal of our project was to create a working heat pipe then conduct a wick analysis comparing the rate of heat transfer of three differently sized wicks. We were not as concerned about our heat pipe operating at maximum efficiency, we just needed to ensure that it was working before proceeding with other tests on different wicks. Since we only had a limited number of tests we could conduct due to time constraints with the testing apparatus, it was more important to keep fluid levels and pipe orientation constant across all trials (in order to create a legitimate analysis) rather than proceeding with future modifications to improve general efficiency.
Trial 1 was conducted using a wick with 22 holes per inch (22 hpi). The blue curve shows the temperature of the heated end of the pipe as a function of time. The orange curve shows the recorded date for the cooler (higher) end of the pipe).
When the old wick was removed, many of its capillaries were saturated with water. We could not possibly know how much water was being lost, so we decided to completely drain the pipe and refill it with new working fluid, to ensure near constant conditions from trial to trial.
Careful note was also made as to the angle of inclination of the pipe during testing, to ensure that it could be returned to this angle for future trials.
We finished off the new pipe by taping the threads with PTFE tape and again heating it from the bottom until vapor exited from the open end. At this point the cap was quickly secured and the new pipe was ready for testing.
Trial 2 was conducted in a similar fashion using a slightly larger wick with 16 holes per inch (16 hpi).
We observed the curved portion of the graph from trial 1 and noticed that it spanned the range t = 300s to t = 570s. We used the below equation to calculate the average rate of heat transfer.
heat transfer rate = ΔT / Δt
This finding confirmed our hypothesis that a smaller wick size would result in a greater rate of heat transfer. The smaller wick provided better capillary action to efficiently return condensed water to the heated end of the pipe for re-evaporation.
We also noticed that the equilibrium temperature for trial 1 was 102°F, 10 degrees higher than the equilibrium temperature for trial 2, which was 92°F. This bolsters our belief that the smaller wick resulted in a more efficient heat pipe, since a greater amount of heat was able to be transferred to the cooler end of the pipe.
We finished by reflecting on some possible sources of error for our experiment. We immediately went to the temperature probes, and noticed that they did not maintain the same amount of contact from one trial to the next. It was difficult to keep the same surface area of the probe in contact with the curved edge of the pipe, which likely skewed some of the temperature readings. We also thought about the wick, and how it could have become bent or deformed when it was inserted into the pipe. Any tight bends or crimps could have negatively affected the ability of the wick to transport condensed water.
Now that we are reaching the conclusion of the project, we have reassessed the project budget and materials list and noticed that the flux, solder, pipe cutter, and one roll of screen, as well as some extra caps and fittings were not used for the heat pipe. These additional materials have been returned, and our savings reflected in our finalized budget below.
Category
|
Cost
|
0.5 in x 5 ft Copper
Type M Pipe
|
$6.76
|
Threaded Steel Cap
|
$1.57
|
Copper Cap
|
$0.67
|
Metal Mesh Screen (3)
|
$25.43
|
PTFE (Teflon) Tape
|
$0.97
|
Threaded Adapter
|
$1.42
|
8% sales tax
|
$2.95
|
TOTAL
|
$39.77
|
By borrowing many materials that we needed to complete the pipe, we were able to save roughly $80.00 off of our original estimate of $119.54.
Heading into week 7, we look forward to potentially conducting a third test with a larger sized wick, to obtain a better idea of how wick size relates to heat transfer. We hope to learn from our mistakes and keep the external variables nearly constant to the other two trials for this upcoming test. We are eager to get started on drafting our final report and analysis for the upcoming week.
-- Alec, Tran, Matt, and Shjon
Thursday, April 28, 2016
Week 5
April 28, 2016
In week 5 we continued to work through some minor problems to set ourselves up for success in the coming weeks.
The first order of business was establishing what was the appropriate amount of working fluid that should be placed in the pipe. In week 4, we tested our pipe filled 25% of the way with water, and it did not function as desired. There was not enough fluid in the pipe, so the water was condensing before it reached the top of the pipe, and therefore was not transferring heat all the way to the cool end of the pipe.
We knew we needed to add more water, but how much more? Over the gap between weeks 4 and 5 we did some research into prior experiments with heat pipes, as well as looked at some professionally crafted heat pipes to see how much of the way they were filled with fluid. What we encountered was a range of answers, all dependent on not only the heat pipe dimensions but on the materials, angle of operation, and functioning temperature. We saw analyses on heat pipes filled up to 85% of the way with water, while some others were as low as 25%.
Immediately, we thought that equations would be needed to describe our application and to choose appropriately from our results. However, we realized that this simply could not be done, since our heat pipe would not be subject to a constant temperature. Furthermore, we would not know what rate the hot air blower would increase in temperature until the experiment was conducted. Thus, we had to use a best estimate for the appropriate amount of working fluid.
Ideally, we would want to use exactly the right amount of water in the pipe for maximum heat transfer efficiency. But our analysis is not concerned with efficiency, only with wick comparison. Thus, if we had enough fluid in the pipe for it to operate as desired, as long as we kept this amount constant through all of the wick tests it would not matter if the amount of fluid indeed proved most effienct for the specific application. We therefore reasoned that 50% of the pipe would be filled with working fluid.
From there we removed the fluid from last trial, refilled the pipe, and went on to heat the water. However, something unusual happened. When the water was being heated in preparation to secure the cap, without any prior warning a large splash of water exited from the open end of the pipe.
We concluded that this must have been caused by heating only one spot on the bottom of the pipe, which caused a gas bubble to form with the hotter water trapped beneath the cooler water. After emptying and refilling the pipe, we again commenced heating, this time moving the heat source uniformly across the bottom half of the pipe. Once a bit of steam was seen exiting the top of the pipe, the cap was secured in place.
Since we tested our pipe the week prior, there were many other groups who were ahead of us in conducting tests of their pipes. Because of this, we did not conduct any tests in week 5. Therefore, we are still unsure if 50% full is the proper amount of working fluid. In week 6, we will test the pipe in its current state, and if that proves successful, we'll go on to test two other wick structures (three in total), reduced from a total of four due to time and resource constraints regarding the testing apparatus.
In the meantime, we were able to cut and assemble the two other wicks. This way, as soon as the proper amount of working fluid is established, we can conduct our tests in quick succession to maximize the time we have to conduct our analysis. The aluminum wire screen was rather rigid and difficult to cut, but we made it work by again using the dowel as a support structure.
Our running hypothesis is that the smaller the wick (holes per inch), the better it will be at conducting heat. Wicks with small openings allow water to condense and be transported more quickly throughout the pipe. We believe this because we have come across the use of sintered metal wicks in a majority of professional applications. Sintered metal is designed to have very small openings which make it easy for water and other fluids to condense and use capillary action as well as gravity to return to the hot end of the pipe. Regarding the wick shown above, with 1/4 inch holes (4 holes per inch), we think that this will not be able to function as a wick. Because the hole size is so large, water will not be able to take advantage of capillary action, and there is not as much surface area upon which to condense. Because of these reasons, we ultimately believe that using this size wick will show no improvement in heat transfer over a heat pipe without a wick entirely.
It will definitely be interesting to tests these hypothesis in the coming weeks, not only to determine if they described the correct trends, but to see by what factor the rate of heat transfer is improved by reducing the size of the holes in the wick. We are going to keep the components as is, using copper for the pipe and water as the working fluid. Although some other pipe materials and working fluids may provide a more efficient transfer of heat, we are more concerned with the wick efficiency analysis in a copper-water heat pipe, the most common type for electronics cooling applications. By focusing on this heat pipe configuration, not only will we be able to conduct a proper analysis but we can produce findings that have relevancy to specific electronics applications.
We look forward to testing and further modifying our heat pipe as well as to beginning an analysis on the rate of heat transfer. We are currently on schedule for a timely delivery of the final report and analysis.
-- Alec, Tran, Matt, and Shjon
In week 5 we continued to work through some minor problems to set ourselves up for success in the coming weeks.
The first order of business was establishing what was the appropriate amount of working fluid that should be placed in the pipe. In week 4, we tested our pipe filled 25% of the way with water, and it did not function as desired. There was not enough fluid in the pipe, so the water was condensing before it reached the top of the pipe, and therefore was not transferring heat all the way to the cool end of the pipe.
We knew we needed to add more water, but how much more? Over the gap between weeks 4 and 5 we did some research into prior experiments with heat pipes, as well as looked at some professionally crafted heat pipes to see how much of the way they were filled with fluid. What we encountered was a range of answers, all dependent on not only the heat pipe dimensions but on the materials, angle of operation, and functioning temperature. We saw analyses on heat pipes filled up to 85% of the way with water, while some others were as low as 25%.
Immediately, we thought that equations would be needed to describe our application and to choose appropriately from our results. However, we realized that this simply could not be done, since our heat pipe would not be subject to a constant temperature. Furthermore, we would not know what rate the hot air blower would increase in temperature until the experiment was conducted. Thus, we had to use a best estimate for the appropriate amount of working fluid.
Ideally, we would want to use exactly the right amount of water in the pipe for maximum heat transfer efficiency. But our analysis is not concerned with efficiency, only with wick comparison. Thus, if we had enough fluid in the pipe for it to operate as desired, as long as we kept this amount constant through all of the wick tests it would not matter if the amount of fluid indeed proved most effienct for the specific application. We therefore reasoned that 50% of the pipe would be filled with working fluid.
From there we removed the fluid from last trial, refilled the pipe, and went on to heat the water. However, something unusual happened. When the water was being heated in preparation to secure the cap, without any prior warning a large splash of water exited from the open end of the pipe.
We concluded that this must have been caused by heating only one spot on the bottom of the pipe, which caused a gas bubble to form with the hotter water trapped beneath the cooler water. After emptying and refilling the pipe, we again commenced heating, this time moving the heat source uniformly across the bottom half of the pipe. Once a bit of steam was seen exiting the top of the pipe, the cap was secured in place.
Since we tested our pipe the week prior, there were many other groups who were ahead of us in conducting tests of their pipes. Because of this, we did not conduct any tests in week 5. Therefore, we are still unsure if 50% full is the proper amount of working fluid. In week 6, we will test the pipe in its current state, and if that proves successful, we'll go on to test two other wick structures (three in total), reduced from a total of four due to time and resource constraints regarding the testing apparatus.
 |
| three different wick sizes will be compared in our analysis |
In the meantime, we were able to cut and assemble the two other wicks. This way, as soon as the proper amount of working fluid is established, we can conduct our tests in quick succession to maximize the time we have to conduct our analysis. The aluminum wire screen was rather rigid and difficult to cut, but we made it work by again using the dowel as a support structure.
 |
| a wooden dowel has proven to be most useful in rolling the wicks |
It will definitely be interesting to tests these hypothesis in the coming weeks, not only to determine if they described the correct trends, but to see by what factor the rate of heat transfer is improved by reducing the size of the holes in the wick. We are going to keep the components as is, using copper for the pipe and water as the working fluid. Although some other pipe materials and working fluids may provide a more efficient transfer of heat, we are more concerned with the wick efficiency analysis in a copper-water heat pipe, the most common type for electronics cooling applications. By focusing on this heat pipe configuration, not only will we be able to conduct a proper analysis but we can produce findings that have relevancy to specific electronics applications.
We look forward to testing and further modifying our heat pipe as well as to beginning an analysis on the rate of heat transfer. We are currently on schedule for a timely delivery of the final report and analysis.
-- Alec, Tran, Matt, and Shjon
Thursday, April 21, 2016
Week 4
April 21, 2016
In week 4 we made big strides in constructing the heat pipe. We started by using the pipe cutter to cut off a 2 ft. long piece of copper pipe to be used as the heat pipe. We chose to use this length because we felt that it would be sufficiently large to serve as a model or prototype for other heat pipes. We were not as much concerned with the pipe itself as we were with conducting a thorough wick analysis.
We then cleaned both ends of the pipe and coated it in flux. The cap piece was placed on one end and soldered, and the adapter on the other end and soldered. Both pieces were soldered securely and the connections were waterproof.
We then went to insert the wick, and encountered a bit of a problem. Initially, we thought that we would simply roll the screen a few times over and slide it right into the pipe. However, this was more difficult than anticipated, since the screen rolled unevenly and had a tendency to crease if bent. In addition, the free ends of the screen had wires poking out in many directions, which made it difficult to simply slide the screen into the pipe.
We did manage to find a solution to this problem. We rolled the screen around a long wooden dowel, which helped to provide structure and prevent creasing. This gave us a tight, uniform roll of screen. By creasing a piece of the screen over the end of the dowel, we could then use the dowel as a sort of ramrod to push the screen role all the way in the pipe, removing the dowel at the end.
PTFE tape was then wrapped around the threaded part of the adapter. This would ensure a secure, watertight seal when the cap was tightened on top. A 1/2 ft. piece of copper pipe was cut off (from scrap pipe) and filled completely with water. This acted as a measuring device so we could fill about 1/4 of the heat pipe with water. We then capped the pipe and inverted it many times to ensure that the entire wick was moist. It is crucial that all parts of the wick are moist before building up pressure in the pipe to ensure that the vapor will condense properly during operation.
Now came the crucial part of the heat pipe construction. We removed the cap, and securing the pipe vertically, heated the bottom end. As soon as vapors were observed at the open end of the pipe, we quickly secured the cap and tightened it with a wrench. The heat pipe was now complete, but we would have to test it to check if it was working properly.
A test rig was assembled as follows. A plate stand was placed on the table top, and a rubber clamp attachment secured to the vertical support rod. The heat pipe was secured in the rubber clamp and tilted to make a roughly 45 degree angle with the horizontal. A hot air blower was placed on the table adjacent to the bottom end of the heat pipe. A glass shield was placed on the other side to prevent the hot air from directly blowing onto the wall behind. One probe of a temperature sensor was placed in contact with the non heated (higher) end of the heat pipe, and secured next to the pipe with electrical tape. The other sensor was left free to be periodically placed in contact with the heated (bottom) end of the heat pipe.
The hot air blower was then turned on and the waiting began. If the heat pipe was working properly, we would expect to see similar temperatures at both the heated and non-heated ends of the pipe. This would signal that the working fluid is effectively transferring heat away from the heated end.
However, our heat pipe was not working as intended. The heated end of the pipe reached roughly 500 degrees F, but the non-heated end never made it above 100 degrees F. But an important observation was made by moving the probe in contact with the heated end to different parts of the pipe. We recognized that when the heated end was roughly 500 degrees F, the middle of the pipe was around 300 degrees F. This meant that our heat pipe was working properly, but the working fluid was condensing well before it reached the top of the pipe.
We recognized that one of two things could be done to remedy this problem. Our first option was to shorten the pipe to roughly half of it's original size. That way the top of the pipe would become the point at which the fluid was condensing. This option would require a bit of work, since the cap and adapter were already soldered to either ends.
Our second option was to insert more working fluid into the pipe. That way, there would be more liquid to vaporize, and it would remain in the vapor stage long enough to reach the top of the pipe before condensing. This was clearly the better option, as we would only have to add more water and recreate the partial vacuum. Unfortunately, we ran out of time, and did not get a chance to test the pipe with additional water added. Over the course of the next week we will be doing research to try to discover if there is an equation that will allow us to find the exact percentage of the pipe that needs to be filled with working fluid for maximum heat transfer.
Hopefully by next lab period, with these changes instituted, we will have a working heat pipe prototype, at which point the analysis of different wicks can begin.
-- Alec, Tran, Matt, and Shjon
In week 4 we made big strides in constructing the heat pipe. We started by using the pipe cutter to cut off a 2 ft. long piece of copper pipe to be used as the heat pipe. We chose to use this length because we felt that it would be sufficiently large to serve as a model or prototype for other heat pipes. We were not as much concerned with the pipe itself as we were with conducting a thorough wick analysis.
We then cleaned both ends of the pipe and coated it in flux. The cap piece was placed on one end and soldered, and the adapter on the other end and soldered. Both pieces were soldered securely and the connections were waterproof.
We then went to insert the wick, and encountered a bit of a problem. Initially, we thought that we would simply roll the screen a few times over and slide it right into the pipe. However, this was more difficult than anticipated, since the screen rolled unevenly and had a tendency to crease if bent. In addition, the free ends of the screen had wires poking out in many directions, which made it difficult to simply slide the screen into the pipe.
We did manage to find a solution to this problem. We rolled the screen around a long wooden dowel, which helped to provide structure and prevent creasing. This gave us a tight, uniform roll of screen. By creasing a piece of the screen over the end of the dowel, we could then use the dowel as a sort of ramrod to push the screen role all the way in the pipe, removing the dowel at the end.
 |
| A wooden dowel was used to provide structure to the rolled screen |
 |
| A piece of copper pipe was used as a measuring tool to fill the heat pipe |
Now came the crucial part of the heat pipe construction. We removed the cap, and securing the pipe vertically, heated the bottom end. As soon as vapors were observed at the open end of the pipe, we quickly secured the cap and tightened it with a wrench. The heat pipe was now complete, but we would have to test it to check if it was working properly.
The hot air blower was then turned on and the waiting began. If the heat pipe was working properly, we would expect to see similar temperatures at both the heated and non-heated ends of the pipe. This would signal that the working fluid is effectively transferring heat away from the heated end.
However, our heat pipe was not working as intended. The heated end of the pipe reached roughly 500 degrees F, but the non-heated end never made it above 100 degrees F. But an important observation was made by moving the probe in contact with the heated end to different parts of the pipe. We recognized that when the heated end was roughly 500 degrees F, the middle of the pipe was around 300 degrees F. This meant that our heat pipe was working properly, but the working fluid was condensing well before it reached the top of the pipe.
We recognized that one of two things could be done to remedy this problem. Our first option was to shorten the pipe to roughly half of it's original size. That way the top of the pipe would become the point at which the fluid was condensing. This option would require a bit of work, since the cap and adapter were already soldered to either ends.
Our second option was to insert more working fluid into the pipe. That way, there would be more liquid to vaporize, and it would remain in the vapor stage long enough to reach the top of the pipe before condensing. This was clearly the better option, as we would only have to add more water and recreate the partial vacuum. Unfortunately, we ran out of time, and did not get a chance to test the pipe with additional water added. Over the course of the next week we will be doing research to try to discover if there is an equation that will allow us to find the exact percentage of the pipe that needs to be filled with working fluid for maximum heat transfer.
Hopefully by next lab period, with these changes instituted, we will have a working heat pipe prototype, at which point the analysis of different wicks can begin.
-- Alec, Tran, Matt, and Shjon
Monday, April 18, 2016
Week 3
April 14, 2016
Now that we had determined the roadmap ahead for our project, week 3 was the time to set everything up so that work could begin. To do that we needed to compile a comprehensive list of materials that would be needed in every aspect of the project. This included not only the pipe and wicks themselves but components such as tinning flux and solder that would be necessary to secure the components.
We also had to begin to consider what materials could be borrowed or rented to try and minimize costs. After talking to the faculty at the Drexel machine shop, we learned that we had access to a wide range of tools and machines, the most important of which being a soldering iron.
We also learned that we could test the heat pipe in lab a few weeks before the end of the term, which will provide time necessary to compile the analysis in addition to the final report. When we test the heat pipe using the test rig, a temperature sensor and insulating tape will be provided by the class advisor, allowing us to collect data without having to purchase those materials.
Through this we were able to eliminate the need to purchase the heat gun and temperature sensor. After purchasing all materials, we also realized we could borrow a pipe cutting tool, so this price will be subtracted from the total cost of materials when it is returned. We decided to make a small design change to the heat pipe, opting to use threaded adapters and caps instead of SharkBite compression caps. Soldering on a threaded adapter will be more secure and reliable over multiple tests.
Another key component to minimization of cost was analyzing shipping rates. After looking at quite a few sites, the shipping rate would only fall to a reasonable price if a) we were ordering hundreds of dollars worth of material or b) we were willing to wait three weeks for shipping. Both of those options were unreasonable, so we decided on paying a bit extra for some materials but eliminating shipping costs by picking up all of the materials from a local hardware retailer.
Because of the tools and materials we were able to borrow and the cost we were able to save from eliminating shipping, we decided to purchase and test four rolls of screen instead of three. In addition, a 5 ft. long copper pipe was purchased, which will be cut into two 2 ft. long pieces and will be used to make two heat pipes. This way the group can use one pipe for testing of the wick (to keep conditions uniform throughout all tests) and then attempt to replicate the findings using the second heat pipe.
While this decision partly arose from the fact that due to a sale the 5 ft. pipe cost just pennies more than the 2 ft. pipe, replicating our results will only serve to bolster the analysis and any conclusions we may reach.
After all materials were purchased, the total came out to be $78.58. The cost of each material is listed in the updated budget below. This total, which is much less than the one detailed in the above budget, is due to the fact that many of the tools and equipment were able to be borrowed. However, the cost some materials, such as solder, an extra set of caps and fittings, Teflon tape, soldering flux, and an extra mesh screen were added into the budget after the decision was made to construct two heat pipes and test four wicks instead of three.
-- Alec, Tran, Matt, and Shjon
Now that we had determined the roadmap ahead for our project, week 3 was the time to set everything up so that work could begin. To do that we needed to compile a comprehensive list of materials that would be needed in every aspect of the project. This included not only the pipe and wicks themselves but components such as tinning flux and solder that would be necessary to secure the components.
Projected Budget
Category
|
Projected Cost
|
Manufacturer
|
Location
|
0.5 in x 5 ft Copper Type M Pipe
|
$6.76
|
Mueller
|
Home Depot
|
Compression pipe cap (2)
|
$13.84
|
SharkBite
|
Home Depot
|
Metal Pipe Cutting Tool
|
$9.97
|
SharkBite
|
Home Depot
|
Heat Insulation Tape
|
$7.28
|
Nashua
|
Home Depot
|
Dual Temperature Heat Gun
|
$21.70
|
Genesis
|
Home Depot
|
Temperature Sensor
|
$26.99
|
Nicety
|
Amazon
|
Aluminum Screen (3)
|
$33.00
|
Saint-Gobain
|
Home Depot
|
Estimated Shipping
|
$0.00
| ||
TOTAL
|
$119.54
|
We also had to begin to consider what materials could be borrowed or rented to try and minimize costs. After talking to the faculty at the Drexel machine shop, we learned that we had access to a wide range of tools and machines, the most important of which being a soldering iron.
We also learned that we could test the heat pipe in lab a few weeks before the end of the term, which will provide time necessary to compile the analysis in addition to the final report. When we test the heat pipe using the test rig, a temperature sensor and insulating tape will be provided by the class advisor, allowing us to collect data without having to purchase those materials.
Through this we were able to eliminate the need to purchase the heat gun and temperature sensor. After purchasing all materials, we also realized we could borrow a pipe cutting tool, so this price will be subtracted from the total cost of materials when it is returned. We decided to make a small design change to the heat pipe, opting to use threaded adapters and caps instead of SharkBite compression caps. Soldering on a threaded adapter will be more secure and reliable over multiple tests.
Another key component to minimization of cost was analyzing shipping rates. After looking at quite a few sites, the shipping rate would only fall to a reasonable price if a) we were ordering hundreds of dollars worth of material or b) we were willing to wait three weeks for shipping. Both of those options were unreasonable, so we decided on paying a bit extra for some materials but eliminating shipping costs by picking up all of the materials from a local hardware retailer.
Because of the tools and materials we were able to borrow and the cost we were able to save from eliminating shipping, we decided to purchase and test four rolls of screen instead of three. In addition, a 5 ft. long copper pipe was purchased, which will be cut into two 2 ft. long pieces and will be used to make two heat pipes. This way the group can use one pipe for testing of the wick (to keep conditions uniform throughout all tests) and then attempt to replicate the findings using the second heat pipe.
While this decision partly arose from the fact that due to a sale the 5 ft. pipe cost just pennies more than the 2 ft. pipe, replicating our results will only serve to bolster the analysis and any conclusions we may reach.
In addition, we are still deciding whether or not to purchase an infrared temperature sensor. The one provided to us in the testing rig may not be compatible with the data collecting software with which we are familiar, and may not be as accurate as we need it to be to establish the correct relationship between the heat transfer variables. If we do eventually choose to purchase this sensor, it will cost $14.63 including shipping (five business days), which will add roughly $8 to the total cost of materials after factoring in the money regained from returning the pipe cutting tool.
Below is a rendered CAD design (to scale) of how the components are going to fit together inside of the heat pipe. The end not shown has water in the bottom and is closed with a soldered cap. The rolled wick will run the length of the pipe and no further; it is shown protruding from the pipe for clarity.
Now that the plan is in place and the materials are in hand, construction of the heat pipe is able to begin. The current goal is to have the entire heat pipe completed by the end of week 5 so that testing can begin starting week 6. Below is a rendered CAD design (to scale) of how the components are going to fit together inside of the heat pipe. The end not shown has water in the bottom and is closed with a soldered cap. The rolled wick will run the length of the pipe and no further; it is shown protruding from the pipe for clarity.
-- Alec, Tran, Matt, and Shjon
Wednesday, April 13, 2016
Week 2
April 7, 2016
Week 2 was the true planning week for the project. While the first week offered a good start by focusing our group on copper/water heat pipes and their use in electronics cooling, the rest of the project has completely changed.
From the outset, we wanted to conduct a materials comparison as a challenge to not only observe physical properties but to describe them with an equation related to each materials effectiveness in transferring heat.
Initially, we thought to compare working fluids, since they are the most easily obtained and replaced. In addition, we would have loads of data on surface tension, viscosity, heat of vaporization, and other crucial properties at our disposal. However, after looking at various fluids in the comparable temperature range of water, which included acetone, methanol, and toluene, we decided that it would be too dangerous to be heating and vaporizing these materials in the workshop environment.
Our group then switched the focus to the pipe metal itself. We could easily compare a few metals such as aluminum, copper, and steel to see which was most effective at transferring heat. The major advantage here is that we already knew what the results of our analysis would be. By using each materials properties to calculate its Merit Number, which describes its effectiveness at certain temperatures, we could easily see that copper was the best metal to use. However, when it came time to compile a materials list, we had trouble finding steel and aluminum pipes that had the correct threading to be secured with an endcap. In addition, those materials could not easily be soldered, thus we decided to change course yet again.
We finally decided upon comparing wicks. The wick is an internal structure that facilitates the transport of condensed vapors back to the bottom of the heat pipe. While they are not necessary for vertical applications (gravity does all of the work), they are needed in horizontal and near-horizontal applications. Many electronics require heat pipes to be installed in this orientation (for example in laptops), so it would be perfectly applicable to our area of focus.
Expensive wicks can be made out of channeled grooves and sintered metal, but we wanted to focus on metal mesh. Metal mesh wicks are cheap, easily inserted and removed, and can be purchased in small quantities, all important factors for our analysis. For our analysis we will be using aluminum screen mesh. This mesh can simply be rolled up and inserted into the heat pipe as long as it is thoroughly wetted and presses up against the walls of the heat pipe. It is important that the screen mesh be wet, else it will not be able to facilitate the movement of condensed vapors back to the bottom of the pipe. This will create a lack of working fluid, causing the heat pipe to not work properly.
However, the main reason we wanted to conduct our analysis on wicks is because we didn't know which would be most effective. When conducting literature study we encountered many tests and experiments detailing the efficiencies of sintered metal wicks and grooved channels, which are the newest, most cutting edge wick technologies. But in all of our study not once did we find testing results on wire mesh wicks.
This is what makes our analysis truly beneficial. Unlike what we expected to do for comparing metals and working fluids, we are not studying some known factor with some end conclusion in mind. We honestly do not know which wick size will turn out to have the fastest rate of heat transfer. This allows us to follow the scientific method by making educated guesses and examining results with nothing to compare them to. Our analysis will then be unique and useful to people who choose to forego expensive commercial technologies in favor of the DIY method of improving and modifying electronic devices.
The next step is to purchase all materials and begin construction of the heat pipe, which should begin by next week. The updated project proposal detailing how we plan to compare the different wicks can be found under the project proposals tab below the original proposal. We remain on schedule as we head into week 3.
-- Alec, Tran, Matt, and Shjon
Week 2 was the true planning week for the project. While the first week offered a good start by focusing our group on copper/water heat pipes and their use in electronics cooling, the rest of the project has completely changed.
From the outset, we wanted to conduct a materials comparison as a challenge to not only observe physical properties but to describe them with an equation related to each materials effectiveness in transferring heat.
Initially, we thought to compare working fluids, since they are the most easily obtained and replaced. In addition, we would have loads of data on surface tension, viscosity, heat of vaporization, and other crucial properties at our disposal. However, after looking at various fluids in the comparable temperature range of water, which included acetone, methanol, and toluene, we decided that it would be too dangerous to be heating and vaporizing these materials in the workshop environment.
Our group then switched the focus to the pipe metal itself. We could easily compare a few metals such as aluminum, copper, and steel to see which was most effective at transferring heat. The major advantage here is that we already knew what the results of our analysis would be. By using each materials properties to calculate its Merit Number, which describes its effectiveness at certain temperatures, we could easily see that copper was the best metal to use. However, when it came time to compile a materials list, we had trouble finding steel and aluminum pipes that had the correct threading to be secured with an endcap. In addition, those materials could not easily be soldered, thus we decided to change course yet again.
We finally decided upon comparing wicks. The wick is an internal structure that facilitates the transport of condensed vapors back to the bottom of the heat pipe. While they are not necessary for vertical applications (gravity does all of the work), they are needed in horizontal and near-horizontal applications. Many electronics require heat pipes to be installed in this orientation (for example in laptops), so it would be perfectly applicable to our area of focus.
Expensive wicks can be made out of channeled grooves and sintered metal, but we wanted to focus on metal mesh. Metal mesh wicks are cheap, easily inserted and removed, and can be purchased in small quantities, all important factors for our analysis. For our analysis we will be using aluminum screen mesh. This mesh can simply be rolled up and inserted into the heat pipe as long as it is thoroughly wetted and presses up against the walls of the heat pipe. It is important that the screen mesh be wet, else it will not be able to facilitate the movement of condensed vapors back to the bottom of the pipe. This will create a lack of working fluid, causing the heat pipe to not work properly.
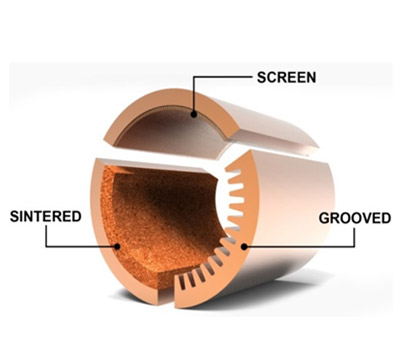 |
| Credit: www.celsiainc.com |
Three common wick structures used in heat pipes
However, the main reason we wanted to conduct our analysis on wicks is because we didn't know which would be most effective. When conducting literature study we encountered many tests and experiments detailing the efficiencies of sintered metal wicks and grooved channels, which are the newest, most cutting edge wick technologies. But in all of our study not once did we find testing results on wire mesh wicks.
This is what makes our analysis truly beneficial. Unlike what we expected to do for comparing metals and working fluids, we are not studying some known factor with some end conclusion in mind. We honestly do not know which wick size will turn out to have the fastest rate of heat transfer. This allows us to follow the scientific method by making educated guesses and examining results with nothing to compare them to. Our analysis will then be unique and useful to people who choose to forego expensive commercial technologies in favor of the DIY method of improving and modifying electronic devices.
The next step is to purchase all materials and begin construction of the heat pipe, which should begin by next week. The updated project proposal detailing how we plan to compare the different wicks can be found under the project proposals tab below the original proposal. We remain on schedule as we head into week 3.
-- Alec, Tran, Matt, and Shjon
Monday, April 4, 2016
Week 1
March 31, 2016
Week 1 of the term marked the beginning of our freshman design project. After forming groups, we decided to begin research into heat pipes and their many forms and applications. While each group member was currently studying a different discipline of engineering, and had a varied base of knowledge and expertise, none of us had any knowledge on heat pipes and how they worked.
We embraced the challenge of starting from a weak knowledge base and building up our understanding through research and collaboration. A majority of the week 1 lab was spent learning about how heat pipes worked, what they were made of, and their many common applications. We were quite surprised at not only their simplicity but how common they were in our daily lives, in everything from laptops to air conditioners. It was amazing that so simple a device, one that required no moving parts or external power, could be still so widely used in the many products we encounter.
After building a sufficient knowledge base, we had to consider the application of our final product. We learned that copper/water heat pipes - copper pipes with water as the working fluid - were not only the cheapest and safest to work with but were the most effective for the temperature ranges in which we would be conducting our analysis. The most common application for copper/water heat pipes is in electronics cooling. Small pipes can be used in laptops to move hot air to the fans where it can exit the device. Larger ones are often used in server farms where the heat needs to be transferred away from the servers to a heat sink (such as water) located elsewhere. One can easily see the usefulness of a heat transport device like a heat pipe when the location of a cooler or other heat sink so close to electronic devices would simply not be feasible.
Because of the minute size and complexity required for prototyping a heat pipe for a laptop or other small device, we decided to construct a larger one (about 3 ft in length) that we would be able to more easily analyze towards the end of the course. In a way, the heat pipe we construct can act as a proof of concept of the effectiveness of a copper/water heat pipe itself, which can be bent and scaled as needed for different applications. It is for this reason that we decided to insert a wick into the interior of the pipe to facilitate condensation. Even though vertically oriented heat pipes can operate on gravity alone, the wick is there to show that the same pipe could be used in nonvertical applications and still work to the same or similar effectiveness as analyzed in the lab.
Periodically throughout the next ten weeks, as well as in the final report and presentation, we will take time to discuss how a certain feature of our prototype can be easily integrated into different electronics applications. While we are simply testing the effectiveness of the materials alone, we want to continue to develop our understanding of how a basic heat pipe can be easily modified to serve a very specific purpose.
Going forward, we hope to continue to further our understanding of the many uses and applications of heat pipes. We will continue to keep you updated on our weekly progress, as well as any other notable findings or events as they might occur on our journey to prototype a copper/water heat pipe. We will also begin to post pictures to document our progress along the way.
-- Alec, Tran, Matt, and Shjon
We embraced the challenge of starting from a weak knowledge base and building up our understanding through research and collaboration. A majority of the week 1 lab was spent learning about how heat pipes worked, what they were made of, and their many common applications. We were quite surprised at not only their simplicity but how common they were in our daily lives, in everything from laptops to air conditioners. It was amazing that so simple a device, one that required no moving parts or external power, could be still so widely used in the many products we encounter.
After building a sufficient knowledge base, we had to consider the application of our final product. We learned that copper/water heat pipes - copper pipes with water as the working fluid - were not only the cheapest and safest to work with but were the most effective for the temperature ranges in which we would be conducting our analysis. The most common application for copper/water heat pipes is in electronics cooling. Small pipes can be used in laptops to move hot air to the fans where it can exit the device. Larger ones are often used in server farms where the heat needs to be transferred away from the servers to a heat sink (such as water) located elsewhere. One can easily see the usefulness of a heat transport device like a heat pipe when the location of a cooler or other heat sink so close to electronic devices would simply not be feasible.
Because of the minute size and complexity required for prototyping a heat pipe for a laptop or other small device, we decided to construct a larger one (about 3 ft in length) that we would be able to more easily analyze towards the end of the course. In a way, the heat pipe we construct can act as a proof of concept of the effectiveness of a copper/water heat pipe itself, which can be bent and scaled as needed for different applications. It is for this reason that we decided to insert a wick into the interior of the pipe to facilitate condensation. Even though vertically oriented heat pipes can operate on gravity alone, the wick is there to show that the same pipe could be used in nonvertical applications and still work to the same or similar effectiveness as analyzed in the lab.
Periodically throughout the next ten weeks, as well as in the final report and presentation, we will take time to discuss how a certain feature of our prototype can be easily integrated into different electronics applications. While we are simply testing the effectiveness of the materials alone, we want to continue to develop our understanding of how a basic heat pipe can be easily modified to serve a very specific purpose.
Going forward, we hope to continue to further our understanding of the many uses and applications of heat pipes. We will continue to keep you updated on our weekly progress, as well as any other notable findings or events as they might occur on our journey to prototype a copper/water heat pipe. We will also begin to post pictures to document our progress along the way.
-- Alec, Tran, Matt, and Shjon
Subscribe to:
Posts (Atom)









