In week 5 we continued to work through some minor problems to set ourselves up for success in the coming weeks.
The first order of business was establishing what was the appropriate amount of working fluid that should be placed in the pipe. In week 4, we tested our pipe filled 25% of the way with water, and it did not function as desired. There was not enough fluid in the pipe, so the water was condensing before it reached the top of the pipe, and therefore was not transferring heat all the way to the cool end of the pipe.
We knew we needed to add more water, but how much more? Over the gap between weeks 4 and 5 we did some research into prior experiments with heat pipes, as well as looked at some professionally crafted heat pipes to see how much of the way they were filled with fluid. What we encountered was a range of answers, all dependent on not only the heat pipe dimensions but on the materials, angle of operation, and functioning temperature. We saw analyses on heat pipes filled up to 85% of the way with water, while some others were as low as 25%.
Immediately, we thought that equations would be needed to describe our application and to choose appropriately from our results. However, we realized that this simply could not be done, since our heat pipe would not be subject to a constant temperature. Furthermore, we would not know what rate the hot air blower would increase in temperature until the experiment was conducted. Thus, we had to use a best estimate for the appropriate amount of working fluid.
Ideally, we would want to use exactly the right amount of water in the pipe for maximum heat transfer efficiency. But our analysis is not concerned with efficiency, only with wick comparison. Thus, if we had enough fluid in the pipe for it to operate as desired, as long as we kept this amount constant through all of the wick tests it would not matter if the amount of fluid indeed proved most effienct for the specific application. We therefore reasoned that 50% of the pipe would be filled with working fluid.
From there we removed the fluid from last trial, refilled the pipe, and went on to heat the water. However, something unusual happened. When the water was being heated in preparation to secure the cap, without any prior warning a large splash of water exited from the open end of the pipe.
We concluded that this must have been caused by heating only one spot on the bottom of the pipe, which caused a gas bubble to form with the hotter water trapped beneath the cooler water. After emptying and refilling the pipe, we again commenced heating, this time moving the heat source uniformly across the bottom half of the pipe. Once a bit of steam was seen exiting the top of the pipe, the cap was secured in place.
Since we tested our pipe the week prior, there were many other groups who were ahead of us in conducting tests of their pipes. Because of this, we did not conduct any tests in week 5. Therefore, we are still unsure if 50% full is the proper amount of working fluid. In week 6, we will test the pipe in its current state, and if that proves successful, we'll go on to test two other wick structures (three in total), reduced from a total of four due to time and resource constraints regarding the testing apparatus.
 |
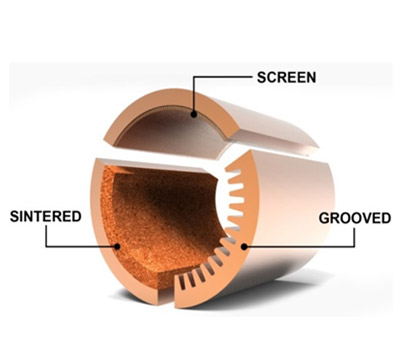
| three different wick sizes will be compared in our analysis |
In the meantime, we were able to cut and assemble the two other wicks. This way, as soon as the proper amount of working fluid is established, we can conduct our tests in quick succession to maximize the time we have to conduct our analysis. The aluminum wire screen was rather rigid and difficult to cut, but we made it work by again using the dowel as a support structure.
 |
| a wooden dowel has proven to be most useful in rolling the wicks |
It will definitely be interesting to tests these hypothesis in the coming weeks, not only to determine if they described the correct trends, but to see by what factor the rate of heat transfer is improved by reducing the size of the holes in the wick. We are going to keep the components as is, using copper for the pipe and water as the working fluid. Although some other pipe materials and working fluids may provide a more efficient transfer of heat, we are more concerned with the wick efficiency analysis in a copper-water heat pipe, the most common type for electronics cooling applications. By focusing on this heat pipe configuration, not only will we be able to conduct a proper analysis but we can produce findings that have relevancy to specific electronics applications.
We look forward to testing and further modifying our heat pipe as well as to beginning an analysis on the rate of heat transfer. We are currently on schedule for a timely delivery of the final report and analysis.
-- Alec, Tran, Matt, and Shjon